Diagnostic Services Ontario Year in Review 2021
Senior Staff and Contact Information
|
Laboratory Medical Director Dr. Wendy Lau, MD, FRCPC |
(416) 313-4433 |
|
Diagnostic Services Manager Tammy Ison, MLT, ART |
(905) 494-5281 |
|
Diagnostic Services Reference Laboratory |
Telephone (905) 494-5295 Fax (905) 494-8131 |
|
Diagnostic Services Website |
Table of contents
- SENIOR STAFF AND CONTACT INFORMATION
- TABLE OF CONTENTS
- FIGURES
- TABLES
- RED CELL SEROLOGY REFERENCE LABORATORY
- REFERRAL SAMPLES
- QUALITY INDICATORS
- DIAGNOSTIC SERVICES UPDATE 2021
- PRESENTATIONS / ABSTRACTS / PUBLICATIONS LISTING
Figures
- Figure 1: Specimens Tested between 2017 and 2021
- Figure 2: Total Number of Clinically Significant Antibodies Identified in Prenatal Samples between 2019 and 2021
- Figure 3: Total Number of Clinically Insignificant Antibodies Identified in Prenatal Samples between 2019 and 2021
- Figure 4: Frequency of Clinically Significant Antibodies Identified in Prenatal Samples between 2019 and 2021
- Figure 5: Total Number of Clinically Significant Antibodies Detected in Patient Reference Samples in the Year 2021
- Figure 6: Frequency of Clinically Significant Antibodies in Patient Reference Samples in the Year 2021
Tables
- Table 1: Specimens Tested between 2017 and 2021
- Table 2: Samples Received Each Month in the Year 2021
- Table 3: Total Number Samples sent from Hospital/Private Laboratories
- Table 4: Total Number Samples with No Antibodies Detected
- Table 5: Total Number of Antibodies Detected in Prenatal Samples
- Table 6: Total Number of Clinically Significant Antibodies Identified in Prenatal Samples between 2019 and 2021
- Table 7: Total Number of Clinically Insignificant Antibodies Identified in Prenatal Samples between 2019 and 2021
- Table 8: Frequency of Clinically Significant Antibodies Identified in Prenatal Samples between 2019 and 2021
- Table 9: Prenatal Combination Antibodies
- Table 10: Perinatal Patient Antibody Titres
- Table 11: Total Number of Clinically Significant Antibodies Detected in Patient Reference Samples in the Year 2021
- Table 12: Total Number of Clinically Insignificant Antibodies Detected in Patient Reference Samples in the Year 2021
- Table 13: Frequency of Clinically Significant Antibodies in Patient Reference Samples in the Year 2021
- Table 14: Number of Investigations for Antibodies to Low Prevalence Antigens
- Table 15: Number of investigations for Antibodies to High Prevalence Antigens
- Table 16: Number of Patient Investigation for a Combination Antibodies in the Year 2021
- Table 17: Antibody Complex Procedures Performed
- Table 18: Genotype procedures referred by Canadian Blood Services
- Table 19: CAP Proficiency Testing Results
- Table 20: IQMH Proficiency Testing Results
Red Cell Serology Reference Laboratory
The Red Cell Serology Reference Laboratory, Ontario Diagnostic Services provides testing for hospitals in the Central Ontario Region and Hamilton Region, and for private laboratories. Hospital patients who are repeatedly transfused may develop red cell antibodies and as a result may have difficulty in tolerating transfusions. Diagnostic Services has specialized and experienced technologists that assist and provide consultation to hospital transfusion medicine laboratories. The Reference Laboratory identifies red cell antibodies and provides transfusion recommendations. Diagnostic Services has a varied selection of specialized procedures and rare reagents to resolve more difficult red cell antibody cases. Staff within our department may collaborate with other references laboratories such as the National Immunohematology Reference Laboratory (NIRL), Grifols Clinical Laboratory & Immunohematology Center and the New York Blood Center.
Diagnostic Services Red Cell Antibody Investigations
In 2021, hospitals have referred 2,674 requests for red cell antibody identification.
Referring hospitals have different capabilities and expertise in resolving red cell antibody investigations. Some hospitals have limited reagents for antibody identification or phenotyping of patient or donor units. Others have access to a wider variety of reagent red cell panels and methods as well as on site immunohematology expertise. A few hospital transfusion medicine laboratories have the resources to resolve the majority of serological problems and send only complex investigations for additional serological or genotyping studies.
Canadian Blood Services, Diagnostic Services provides consultation and testing support including antibody investigation, advanced or alternative techniques where required, and recommendations for compatibility testing methods and selection of appropriate donor unit phenotypes if necessary.
Reporting may include interim, final and supplemental reports, depending on the urgency of the testing, the need for patient transfusion and the complexity of investigation.
Testing Performed
The Red Cell Reference Laboratory routinely performs the following tests:
- ABO/Rh blood type and discrepancy investigations (if required)
- Screen for red blood cell antibodies
- Antibody Identification, if antibodies are detected
- Phenotyping (patient)
- Direct Antiglobulin Test
- Elution and Adsorption
- Other tests and techniques, as required.
Serological samples submitted for testing are categorized into either “Prenatal Samples” or “Patient Samples”. Canadian Blood Services – Ontario Page 6 of 27 Diagnostic Services – (2021)
Antibody Screening and identification is routinely performed using a Gel Card testing methodology. A combination of Gel Card testing methodology and indirect antiglobulin tube testing using saline, enzymes or PEG enhancement are the most common antibody identification methods.
The laboratory also coordinates Red Cell Genotyping referral through the Canadian Blood Services National Immunohematology Reference Laboratory (NIRL). The Brampton laboratory is also responsible for maintaining the Central Ontario Sickle Cell Registry.
Table 1: Specimens Tested between 2017 and 2021
|
Specimen Type |
Test Type |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|---|
|
Patient Samples for Red Cell Serology Reference and Prenatal Samples |
ABO Resolutions |
51 |
80 |
82 |
97 |
377 |
|
Antibody investigations - pretransfusion |
708 |
676 |
678 |
869 |
984 |
|
|
Antibody investigations - prenatal |
277 |
329 |
410 |
548 |
645 |
|
|
Phenotyping (number of antigens) |
2,776 |
2,874 |
3,212 |
3,480 |
4,249 |
|
|
Total # of Specimens Tested |
3,812 |
3,959 |
4,382 |
4,994 |
6,255 |
|
|
Total # of Patients Tested |
716 |
670 |
987 |
1,107 |
2,674 |
|
Table 2: Samples Received Each Month in the Year 2021
| Sample Type | January-21 | February-21 | March-21 | April-21 | May-21 | June-21 | July-21 | August-21 | September-21 | October-21 | November-21 | December-21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | 112 | 180 | 140 | 147 | 133 | 145 | 125 | 130 | 180 | 162 | 101 | 105 |
| Prenatal | 75 | 53 | 88 | 64 | 114 | 84 | 104 | 65 | 121 | 111 | 82 | 53 |
The sample total for antibody investigations is 2,674 samples in 12 months or an average of 223 samples per month.
Hospital/Private Laboratory Referrals:
Samples referred into the Brampton Diagnostic Services Laboratory are from:
- 62 Health Care Facilities
- 3 Private Labs (Alpha, LifeLabs and Med-Health)
Private Labs are referring in primarily prenatal samples (94%) with only 6% patient samples for antibody investigation.
Table 3: Total Number Samples sent from Hospital/Private Laboratories
|
Alpha Laboratories Inc. |
Prenatal |
73 |
76 |
|
|
Patient |
3 |
|
||
|
LifeLabs |
Prenatal |
110 |
114 |
|
|
Patient |
4 |
|
||
|
Med-Health Laboratories Inc. |
Prenatal |
22 |
28 |
|
|
Patient |
6 |
|
||
|
|
|
|
218 |
Totals |
|
|
|
|
205 |
Prenatal |
|
|
|
|
13 |
Patient |
The hospital laboratories are referring in a combination of patient and prenatal samples for investigation.
Table 4: Total Number Samples with No Antibodies Detected
| Prenatal | Patient | Total |
|---|---|---|
| 110 | 90 | 200 |
Table 5: Total Number of Antibodies Detected in Prenatal Samples
|
Multiple Antibody Combinations Identified |
Number of Prenatal Multiple Antibody Investigation in (Current Year) |
|---|---|
|
Anti-C Anti-e |
1 |
|
Anti-E, Anti-Lua |
1 |
|
Anti-Fya, Anti-Jka |
1 |
|
Anti-D, Anti-E |
1 |
|
Anti-E, Anti-Lua |
1 |
|
Anti-Fya, Anti-Jka |
1 |
|
Anti-Jka, Anti-Mia |
1 |
|
Anti-c, Autoantibody |
1 |
|
Anti-C, Anti-D |
1 |
|
Autoantibody, Cold Agglutinin |
1 |
|
Anti-C, Anti-e |
1 |
|
Anti-Lea, Anti-M |
1 |
|
Anti-E, Anti-c |
3 |
|
Anti-D, Anti-G |
1 |
|
Anti-D, Anti-G |
1 |
|
Anti-M, Anti-Wra |
1 |
|
Anti-Fya, Anti-Lub |
1 |
|
Anti-c, Anti-E, Anti-Fya |
1 |
|
Anti-D, Anti-G |
4 |
|
Anti-D, Antibody to an HLA related antigen |
1 |
|
Anti-c, Anti-Jka |
1 |
|
Anti-Wra, Antibody to an HLA related antigen |
1 |
|
Anti-C, Anti-D, Anti-Jka |
1 |
|
Anti-E, Anti-K, Anti-M |
1 |
|
Anti-C, Anti-G |
3 |
|
Anti-Jkb, Anti-M |
|
Figure 2: Total Number of Clinically Significant Antibodies Identified in Prenatal Samples between 2019 and 2021
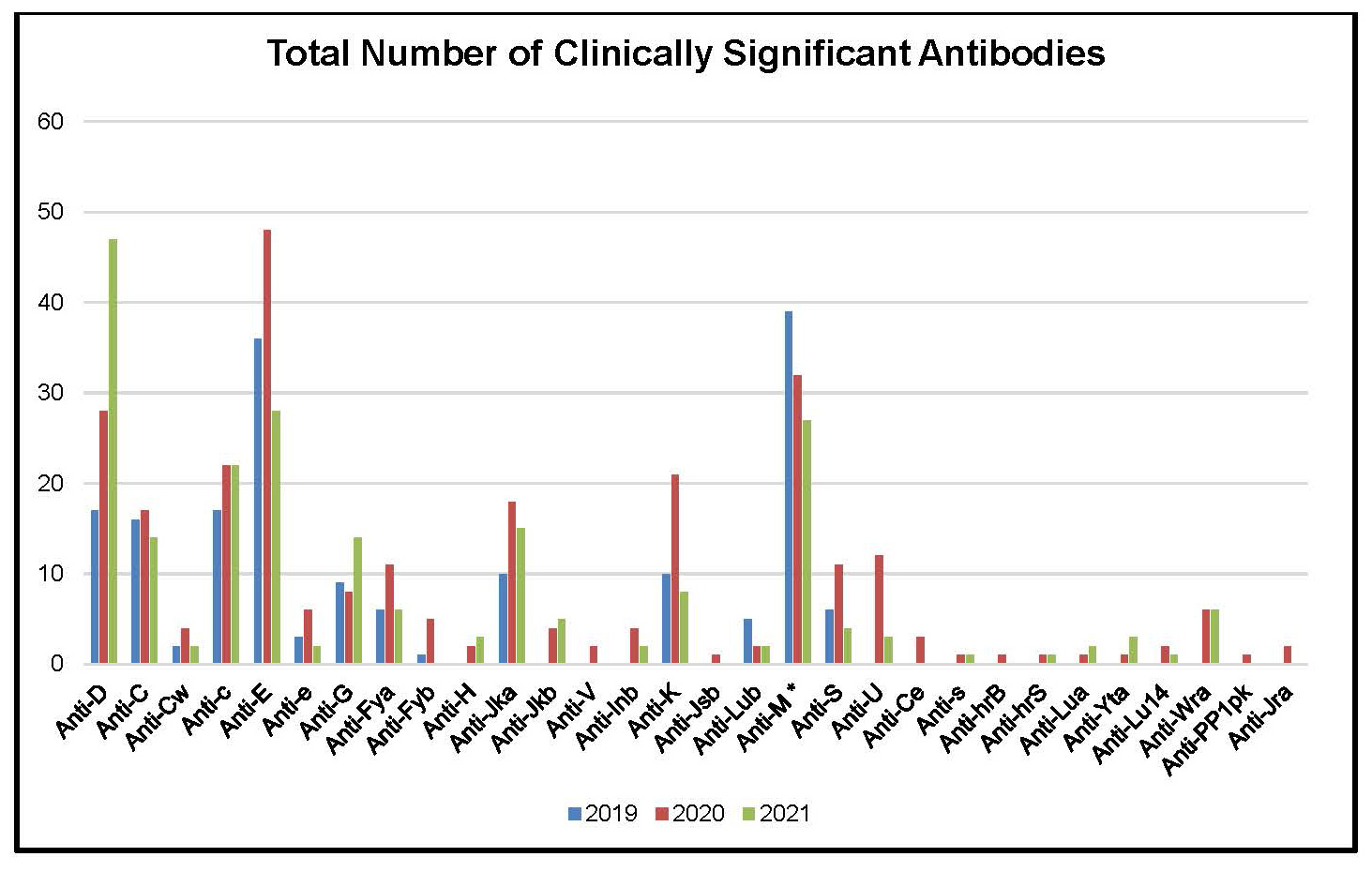
Table 6: Total Number of Clinically Significant Antibodies Identified in Prenatal Samples between 2019 and 2021
|
Number of Prenatal Investigation for each Antibody |
|||
|---|---|---|---|
|
Clinically Significant Antibodies -Identified |
2019 |
2020 |
2021 |
|
Anti-D |
17 |
28 |
47 |
|
Anti-C |
16 |
17 |
14 |
|
Anti-Cw |
2 |
4 |
2 |
|
Anti-c |
17 |
22 |
22 |
|
Anti-E |
36 |
48 |
28 |
|
Anti-e |
3 |
6 |
2 |
|
Anti-G |
9 |
8 |
14 |
|
Anti-Fya |
6 |
11 |
6 |
|
Anti-Fyb |
1 |
5 |
0 |
|
Anti-H |
0 |
2 |
3 |
|
Anti-Jka |
10 |
18 |
15 |
|
Anti-Jkb |
0 |
4 |
5 |
|
Anti-V |
0 |
2 |
0 |
|
Anti-Inb |
0 |
4 |
2 |
|
Anti-K |
10 |
21 |
8 |
|
Anti-Jsb |
0 |
1 |
0 |
|
Anti-Lub |
5 |
2 |
2 |
|
Anti-M * |
39 |
32 |
27 |
|
Anti-S |
6 |
11 |
4 |
|
Anti-U |
0 |
12 |
3 |
|
Anti-Ce |
0 |
3 |
0 |
|
Anti-s |
0 |
1 |
1 |
|
Anti-hrB |
0 |
1 |
0 |
|
Anti-hrS |
0 |
1 |
1 |
|
Anti-Lua |
0 |
1 |
2 |
|
Anti-Yta |
0 |
1 |
3 |
|
Anti-Lu14 |
0 |
2 |
1 |
|
0 |
6 |
6 |
|
|
Anti-PP1pk |
0 |
1 |
0 |
|
Anti-Jra |
0 |
2 |
0 |
|
Total |
177 |
277 |
218 |
Figure 3: Total Number of Clinically Insignificant Antibodies Identified in Prenatal Samples between 2019 and 2021

Table 7: Total Number of Clinically Insignificant Antibodies Identified in Prenatal Samples between 2019 and 2021
|
Clinically Insignificant Antibodies |
2019 |
2020 |
2021 |
|---|---|---|---|
|
Anti-Lea |
14 |
5 |
6 |
|
Anti-Leb |
8 |
6 |
3 |
|
Anti-P1 |
3 |
1 |
2 |
|
Autoantibody |
8 |
35 |
70 |
|
Antibody to HLA Antigens |
9 |
1 |
11 |
|
11 |
3 |
4 |
|
|
Unidentified |
2 |
5 |
3 |
|
Passive Anti-D |
43 |
104 |
88 |
|
Antibody to low prevalence antigen |
1 |
1 |
0 |
|
TOTAL: Clinically Insignificant Antibodies |
99 |
161 |
187 |
Figure 4: Frequency of Clinically Significant Antibodies Identified in Prenatal Samples between 2019 and 2021
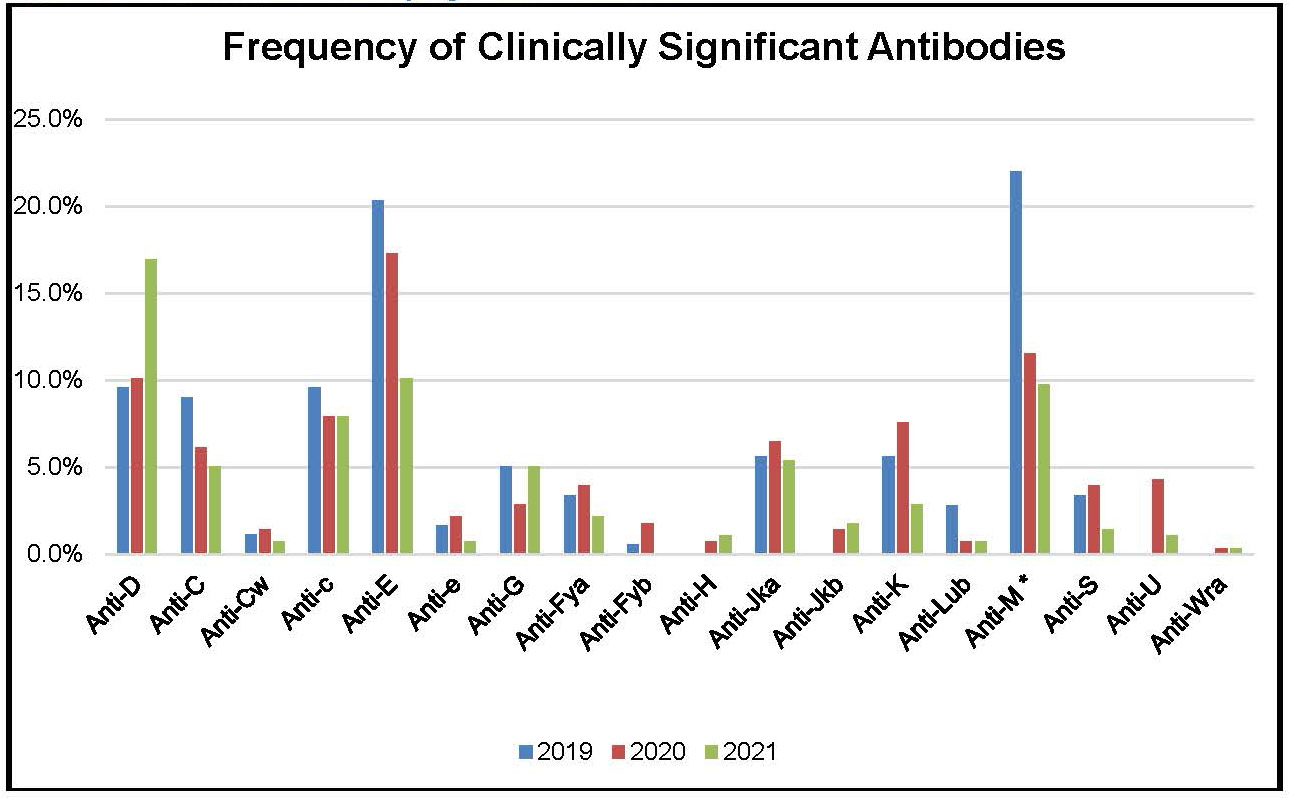
Table 8: Frequency of Clinically Significant Antibodies Identified in Prenatal Samples between 2019 and 2021
|
Antibodies |
2019 |
2020 |
2021 |
|---|---|---|---|
|
Anti-D |
9.6% |
10.1% |
17.0% |
|
Anti-C |
9.0% |
6.1% |
5.1% |
|
Anti-Cw |
1.1% |
1.4% |
0.7% |
|
Anti-c |
9.6% |
7.9% |
7.9% |
|
Anti-E |
20.3% |
17.3% |
10.1% |
|
Anti-e |
1.7% |
2.2% |
0.7% |
|
Anti-G |
5.1% |
2.9% |
5.1% |
|
Anti-Fya |
3.4% |
4.0% |
2.2% |
|
Anti-Fyb |
0.6% |
1.8% |
0.0% |
|
Anti-H |
0.0% |
0.7% |
1.1% |
|
Anti-Jka |
5.6% |
6.5% |
5.4% |
|
Anti-Jkb |
0.0% |
1.4% |
1.8% |
|
Anti-K |
5.6% |
7.6% |
2.9% |
|
Anti-Lub |
2.8% |
0.7% |
0.7% |
|
22.0% |
11.6% |
9.7% |
|
|
Anti-S |
3.4% |
4.0% |
1.4% |
|
Anti-U |
0.0% |
4.3% |
1.1% |
|
Anti-Wra |
0.0% |
0.4% |
0.4% |
Table 9: Prenatal Combination Antibodies
Summary: In 2021 there were 46 antibody investigations for multiple antibodies with 36 different antibody combinations examined.
|
Multiple Antibody Combinations Identified |
Number of Prenatal Multiple Antibody Investigation in (Current Year) |
|---|---|
|
Anti-Jsb, Anti-E |
1 |
|
Anti-s, Anti-D |
1 |
|
Anti-c, Anti-Inb |
1 |
|
Anti-Kpa, Anti-D |
3 |
|
Anti-N, Anti-E |
1 |
|
Anti-c, Anti-Jka |
1 |
|
Anti-hrS, Autoantibody |
1 |
|
Anti-C, Anti-E, anti-D |
1 |
|
anti-M, Anti-c |
2 |
|
Anti-C, Anti-Lea |
1 |
|
Anti-Fyb, Anti-M |
1 |
|
anti-M, anti-Jkb |
2 |
|
Anti-S, Anti-c |
1 |
|
Anti-G, Autoantibody |
1 |
|
Anti-G, Anti-C |
2 |
|
Anti-Leb, Anti-c |
1 |
|
Anti-G, Anti-c |
2 |
|
Anti-Jka, Anti-Lea |
2 |
|
Anti-Jka, Anti-E |
2 |
|
Anti-Jka, Anti-S, Autoantibody |
1 |
|
Anti-c, Anti-Jra |
1 |
|
Anti-Jkb, autoantibody |
1 |
|
Anti-Lea, Anti-Leb |
3 |
|
Anti-Wra, Anti-K, Anti-E |
1 |
|
Anti-K, Autoantibody |
1 |
|
Anti-S, Anti-Lea |
1 |
|
Anti-S, Anti-D |
1 |
|
Anti-C, Anti-M |
1 |
|
Anti-K, Anti-Fyb, Anti-E |
1 |
|
Anti-D, Anti-Cw |
1 |
|
Anti-S, Anti-U |
1 |
|
Anti-S, Anti-Lea |
1 |
|
1 |
|
|
Anti-Jkb, Anti-K |
1 |
|
Anti-E, Cold antibody |
1 |
|
Anti-c, Anti-Lua |
1 |
Table 10: Perinatal Patient Antibody Titres
|
Antibody |
Critical Level |
Non-Critical Level |
Non-Critical to Critical |
|---|---|---|---|
|
Anti-D |
2 |
4 |
1 |
|
Anti-C |
0 |
1 |
0 |
|
Anti-c |
0 |
1 |
0 |
|
Anti-E |
0 |
2 |
0 |
|
Anti-M |
0 |
1 |
0 |
|
anti-C, anti-G |
0 |
3 |
1 |
|
1 |
0 |
0 |
|
|
anti-Jka |
0 |
2 |
0 |
|
Anti-Jkb |
0 |
1 |
0 |
|
anti-S |
0 |
1 |
0 |
Figure 5: Total Number of Clinically Significant Antibodies Detected in Patient Reference Samples in the Year 2021
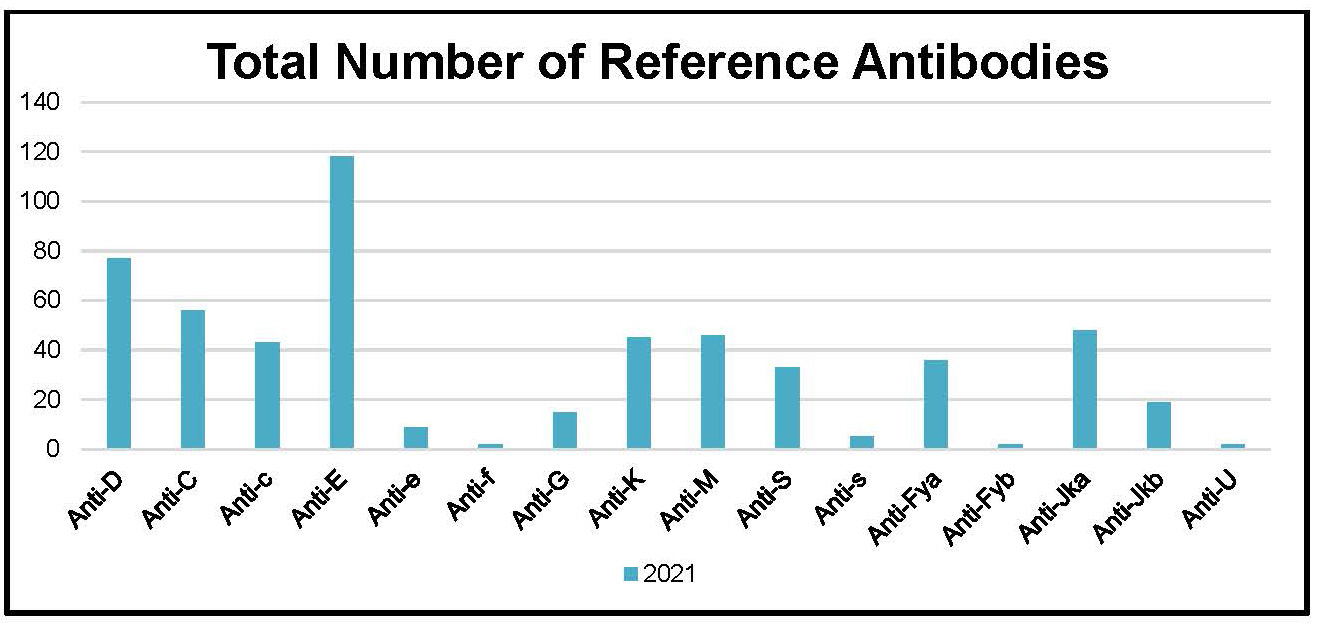
Table 11: Total Number of Clinically Significant Antibodies Detected in Patient Reference Samples in the Year 2021
|
Common Clinically Significant Antibodies in Patient Reference Samples |
2021 |
|---|---|
|
Anti-D |
77 |
|
Anti-C |
56 |
|
Anti-c |
43 |
|
Anti-E |
118 |
|
Anti-e |
9 |
|
Anti-f |
2 |
|
Anti-G |
15 |
|
Anti-K |
45 |
|
Anti-M |
46 |
|
Anti-S |
33 |
|
Anti-s |
5 |
|
Anti-Fya |
36 |
|
Anti-Fyb |
2 |
|
48 |
|
|
Anti-Jkb |
19 |
|
Anti-U |
2 |
|
TOTAL: |
556 |
Table 12: Total Number of Clinically Insignificant Antibodies Detected in Patient Reference Samples in the Year 2021
|
Clinically Insignificant Antibodies |
2021 |
|---|---|
|
Anti-A1 |
9 |
|
Anti-Lea |
15 |
|
Anti-Leb |
5 |
|
Anti-McCa |
2 |
|
Anti-N |
3 |
|
Anti-P1 |
5 |
|
Anti-Rg |
1 |
|
Autoantibody |
351 |
|
Antibody to HLA Antigens |
33 |
|
1 |
|
|
Cold Agglutinin |
37 |
|
Unidentified |
5 |
|
TOTAL: Clinically Insignificant Antibodies |
427 |
Figure 6: Frequency of Clinically Significant Antibodies in Patient Reference Samples in the Year 2021
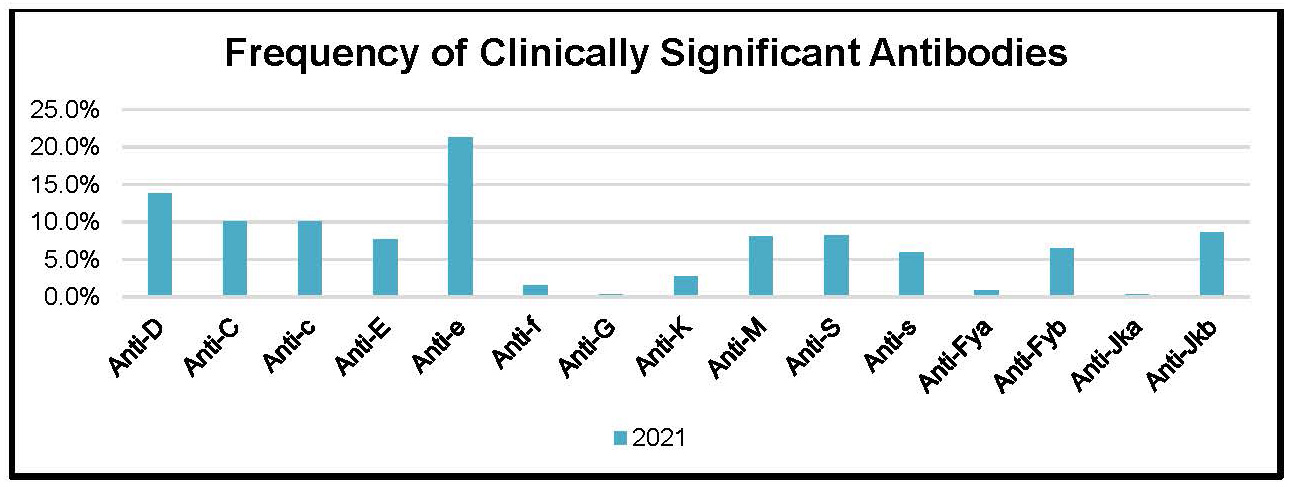
Table 13: Frequency of Clinically Significant Antibodies in Patient Reference Samples in the Year 2021
|
Frequency of Clinically Significant Antibodies |
2021 |
|---|---|
|
Anti-D |
13.8% |
|
Anti-C |
10.1% |
|
Anti-c |
10.1% |
|
Anti-E |
7.7% |
|
Anti-e |
21.2% |
|
Anti-f |
1.6% |
|
Anti-G |
0.4% |
|
Anti-K |
2.7% |
|
Anti-M |
8.1% |
|
Anti-S |
8.3% |
|
Anti-s |
5.9% |
|
0.9% |
|
|
Anti-Fyb |
6.5% |
|
Anti-Jka |
0.4% |
|
Anti-Jkb |
8.6% |
Table 14: Number of Investigations for Antibodies to Low Prevalence Antigens
|
Antibody |
Number Identified |
|---|---|
|
Anti-Cw |
2 |
|
Anti-Dia |
3 |
|
Anti-Jsa |
3 |
|
Anti-Lua |
4 |
|
Anti-VS |
1 |
|
Anti-Kpa |
2 |
|
Anti-Mia |
7 |
|
Anti-V |
3 |
|
Anti-Dantu |
1 |
|
Anti-He |
1 |
|
6 |
|
|
Anti-Cob |
3 |
|
Anti-Wra |
5 |
|
Antibodies to low prevalence antigen |
41 |
Table 15: Number of investigations for Antibodies to High Prevalence Antigens
|
Antibody |
Number Identified |
|---|---|
|
Anti-Ch |
1 |
|
Anti-H |
4 |
|
Anti-hrB |
1 |
|
Anti-Jk3 |
2 |
|
Anti-Fy3 |
1 |
|
Anti-Kna |
2 |
|
Anti-Kpb |
2 |
|
Anti-hrS |
2 |
|
Anti-Coa |
3 |
|
Anti-LW |
3 |
|
anti-Ge2 |
4 |
|
Anti-Dib |
2 |
|
Anti-Inb |
1 |
|
Anti-Lub |
3 |
|
Anti-PP1Pk |
1 |
|
Anti-U |
2 |
|
Anti-Joa |
2 |
|
Anti-Jsb |
2 |
|
Anti-Yta |
3 |
|
1 |
|
|
Anti-Hr |
1 |
|
Anti-JMH |
2 |
|
Antibodies to high prevalence antigen |
25 |
Table 16: Number of Patient Investigation for a Combination Antibodies in the Year 2021
|
Multiple Antibodies Detected |
2021 |
Multiple Antibodies Detected |
2021 |
|---|---|---|---|
|
Anti-C, Anti-S |
1 |
Anti-D, Anti-E |
5 |
|
Anti-Sda, Warm Autoantibody, Cold Agglutinin |
1 |
Anti-D, Anti-C, Anti-E, Anti-S, Anti-K, Anti-Fya |
1 |
|
Anti-Wra, Antibody to HLA related antigen |
1 |
Anti-D, Anti-C |
10 |
|
Anti-E, Anti-c, Anti-Jka |
2 |
Anti-E, Anti-Cob |
1 |
|
Anti-E, Anti-VS, Anti-Jka, Anti-V, Anti-Jsa, Autoantibody |
1 |
Anti-c, Anti-Jka |
1 |
|
Anti-E, Autoantibody |
15 |
Anti-S, Autoantibody |
3 |
|
Anti-E, Anti-S, Anti-K |
1 |
Anti-C, Anti-S, Anti-K |
1 |
|
Anti-K, Autoantibody |
8 |
Anti-E, Anti-M, Anti-Jka |
1 |
|
Anti-E, Anti-S, Anti-Jka, Autoantibody |
1 |
Anti-c, Anti-S, Autoantibody |
2 |
|
Anti-C, Anti-K, Anti-Jkb, Anti-Jsa, Autoantibody |
1 |
Anti-N, Warm Autoantibody |
1 |
|
Anti-E, Anti-M |
1 |
Anti-D, Anti-Ge2 |
1 |
|
Anti-M, Anti-Fya, Antibody To HLA related antigen |
1 |
Anti-Leb, Anti-Jka |
1 |
|
Anti-E, anti-c, Anti-Jka |
1 |
Anti-E, Anti-Wra, Antibody to HLA related antigen |
1 |
|
Anti-E, Anti-Lua, Anti-Jka |
1 |
Anti-E, Anti-Cw |
1 |
|
Anti-E, Anti-Lua, Antibody to HLA related antigen |
1 |
Anti-C, Autoantibody |
3 |
|
Anti-C, Anti-E, Anti-Kpa, Anti-Wra, Autoantibody |
1 |
Anti-Jka, Autoantibody |
5 |
|
Anti-C, Anti-Fya, Anti-Jkb |
1 |
Anti-E, anti-s, Anti-Jka |
1 |
|
Anti-Lua, Autoantibody |
1 |
Anti-C, Anti-S, Anti-Kpa, Autoantibody |
1 |
|
Anti-C, Anti-E, Anti-S, Anti-Fya, Anti-Jkb |
1 |
Anti-Fya, Antibody to HLA related antigen |
1 |
|
Anti-Cw, Anti-Fya |
2 |
Anti-E, anti-c |
4 |
|
Anti-K, Anti-Fya, Anti-Jka, Antibody to HLA related antigen |
1 |
Anti-C, Anti-e, Anti-Jka, Autoantibody |
1 |
|
Anti-E, Anti-K |
1 |
Anti-c, Anti-S, Autoantibody |
1 |
|
Anti-C, Anti-E, Anti-Lea, Anti-Fya, Anti-Jkb |
1 |
Anti-D, Anti-C, Anti-P1 |
1 |
|
Anti-E, Anti-V |
1 |
Anti-K, Anti-Ch |
1 |
|
Anti-C, Anti-e, Autoantibody |
2 |
Anti-Fya, Anti-Jka, Autoantibody |
3 |
|
Anti-E, Anti-K, Autoantibody |
1 |
Anti-E, Anti-Fya, Anti-Wra |
1 |
|
Anti-E, Anti-K, Anti-Fya |
2 |
Anti-C, Anti-e, |
2 |
|
Anti-c, Autoantibody |
1 |
Anti-c, Anti-S, Anti-Fyb, Anti-Mia |
1 |
|
Anti-He, Anti-Wra, Autoantibody |
1 |
Anti-D, Anti-G |
3 |
|
Anti-E, Anti-c, Anti-M, Cold Agglutinin |
1 |
Anti-Fya, Autoantibody |
1 |
|
Anti-E, Anti-c, Anti-M, Autoantibody |
2 |
Anti-s, Anti-Fya, Anti-Jkb, Autoantibody |
1 |
|
Anti-E, Cold Aggultinin |
1 |
Anti-E, Anti-S, Anti-K, Anti-Fya-, Anti-Jkb, Anti-Cob |
1 |
|
Anti-D, Anti-Lua, Autoantibody |
1 |
Anti-E, Anti-S, Anti-K, Autoantibody |
1 |
|
Anti-E, Anti-S, Anti-K |
1 |
Anti-Lea, Anti-Fya, Cold Agglutinin |
1 |
|
Anti-E, Anti-c, Anti-S |
2 |
Anti-E, Anti-c, Anti-Fya |
1 |
|
Anti-E, Anti-Kpa |
1 |
Anti-E, anti-c, Anti-Jkb |
1 |
|
Anti-S, Anti-Ch |
1 |
Anti-M, Anti-Fya, Antibody to an HLA related antigen |
1 |
|
Anti-f, Anti-Fya |
1 |
Anti-E, Anti-c, Autoantibody |
1 |
|
1 |
Anti-N, Anti-S, Anti-Fya |
1 |
|
|
Anti-E, Anti-Lua |
2 |
Anti-C, Anti-G |
2 |
|
Anti-M, Anti-Jkb |
1 |
Anti-D, Anti-C, Anti-G |
1 |
Summary: In 2021 there were 141 antibody investigations for multiple antibodies with 80 different antibody combinations examined.
Table 17: Antibody Complex Procedures Performed
|
Procedures |
Number of Prenatal Samples |
Number of Referral Samples |
|---|---|---|
|
Autoadsorption |
14 |
40 |
|
31 |
94 |
|
|
Elution |
76 |
581 |
|
Direct Coombs |
720 |
2567 |
Referral Samples
1.2. Red Cell Genotyping
The BioArray BeadChip™ test system has been installed and validated in the Diagnostic Services Laboratory in Edmonton for RHD genotype testing used for the identification of RHD variants. The Edmonton CBS laboratory is accredited by the College of Physicians and Surgeons of Alberta (CPSA). Any patient samples requiring extended red cell genotype testing other than for D variant are referred to the National Immunohematology Reference Laboratory (NIRL) in Brampton. NIRL performs extended genotype testing using the Progenika ID Core XT™ assay. If genotype test results are required urgently, testing results can be provided within 24 hours of the sample receipt.
Table 18: Genotype procedures referred by Canadian Blood Services
|
Number of Ontario Genotype Procedures 2021 |
|
|---|---|
|
Number |
|
|
RHD Genotype Procedures |
331 |
|
Non-RHD Genotyping |
1737 |
1.3. Red Cell Serological Reference Testing
The National Immunohematology Reference Laboratory (NIRL) in Brampton is a highly specialized laboratory that focuses its attention on the identification and resolution of exceedingly complex red cell transfusion-related problems. The laboratory is accredited by Accreditation Canada Diagnostics.
Quality Indicators
The laboratories monitor many quality indicators and the two which are most relevant to this document are turnaround times and rejected specimens which are presented below.
1.4. Turnaround Times
To ensure timely reporting of patient test results, Canadian Blood Services monitors turnaround time (TAT) from when the specimen is received at Canadian Blood Services in Brampton to the time when the results are available. Since monitoring of this quality indicator began in 2008, the percentage of specimens has consistently exceeded the predefined TAT threshold of 75% of samples to be tested and reported within 5 days of receipt. In 2021, 87% of the samples received were tested and reported within 5 days of receipt. Samples whose testing exceed the expected TAT are usually those where complex clinically significant antibodies are detected or where a referral to the National Immunohematology Reference Laboratory for additional investigation or genotype testing is required.
1.5. Rejected Specimens
The laboratory reserves the right to refuse improperly labelled specimens. Consistent practices for specimen rejection are employed across CBS. The laboratory takes measures to maintain specimen integrity during the process of following up on the receipt of an improperly identified specimen. The high number of specimens received by the laboratory makes it impossible to positively identify specimens that are not clearly labelled in accordance with standard specimen identification criteria. The specimen rejection rate in 2021 was 1.1% which is decreased from the 1.4% in 2020.
1.6. Proficiency Testing
- College of American Pathologists Survey Participation
This summary is based on all the College of American Pathologists (CAP) survey reports from the Brampton Diagnostic Services site. This summary includes all the blood group serology processes.
Table 19: CAP Proficiency Testing Results
|
Brampton Diagnostic Site (Red Cell) |
2019 CAP Proficiency Results |
2020 CAP Proficiency Results |
2021 CAP Proficiency Results |
|---|---|---|---|
|
ABO/Rh Type |
100% |
100% |
100% |
|
Antibody Titre |
100% |
100% |
100% |
|
Antibody Identification |
100% |
100% |
100% |
|
100% |
100% |
100% |
|
|
Direct Coombs C3 |
100% |
100% |
100% |
|
Direct Coombs IgG |
100% |
100% |
100% |
|
Unexpected Antibody Detected |
100% |
100% |
100% |
Table 20: IQMH Proficiency Testing Results
|
Brampton: TMED |
Kit # |
Date Results Received |
Results |
|---|---|---|---|
|
Brampton |
2021-03-09 |
100% |
|
|
Brampton |
TMED-2106-Advanced |
2021-07-01 |
100% |
|
Brampton |
TMED-2109-Advanced |
2021-09-14 |
100% |
Updates pertain to all Diagnostic Services sites within Canadian Blood Services: Vancouver, Edmonton, Winnipeg, and Brampton
|
ALL |
NEO Iris Analyzer implemented April to June 2021. Eight NEO Instruments in Diagnostic Services were replaced with the next generation NEO IRIS instrument. NEO Iris performs ABO/RH and antibody testing.
|
|
|---|---|---|
|
Edmonton |
Edmonton DS obtained the CPSA 4-year accreditation on 20021-02-25. |
|
|
Edmonton |
Transfer of HEA and RHCE genotype testing to Brampton, 2021-10-01 |
|
|
Vancouver |
Awarded CAP Accreditation Dec 2021. |
|
|
Vancouver |
CPSBC – DAP ISO 15189 Audit. ISO 15189 accreditation pending final acceptance. |
|
|
Winnipeg |
Preparation for implementation of the Canadian Blood Services satellite Lab at St Boniface Hospital in March 2022. The Lab will act as a contingency site for services delivered by Winnipeg Diagnostic Services. |
|
|
Implementation of equipment in NPIRL – Multisizer 3 (cell counter) and thermocyclers |
||
|
Winnipeg |
Management of supplies, inventory and testing to ensure provision of services are not impacted during supply chain issues experienced in a pandemic. |
|
|
Winnipeg |
eTraceLine environments (perinatal and Crossmatch) were merged to allow better efficiency and ease of use for the labs noe that staff are cross trained. |
|
|
Winnipeg |
Project to implement HistoTrac and replace the access database currently used as the LIS began in Winnipeg in 2021. Projected implementation is December 2022. |
|
Presentations / Abstracts / Publications Listing
|
Lhevinne Ciurcovich, Lynnette Beaudin, Arianne Fuellos, Balkar Gill, Ilona Resz, Debra Lane, Judith Hannon, Gwen Clarke, Melanie Bodnar. Comparison of Manual SIAT vs Automated Solid Phase Methodology for Perinatal Antibody Titration. Poster, CSTM 2021 |
|
|
Lhevinne Ciurcovich1, Sarah Manfredi2, Sarah Buchko2, Darlene Mueller2, Michelle Wong2, Mohammad Bahmanyar2, MatthewYan1, Gwen Clarke1. Anti-Ina Implicated in Hemolytic Disease of the Fetus and Newborn in an Indigenous Woman. Poster, CSTM 2021
|
|
|
Lhevinne Ciurcovich, Gwen Clarke, Matthew Yan. A Case of ABO Chimerism in a Perinatal Patient. Poster, CSTM 2021 |
|
|
Lhevinne Ciurcovich. Cell-Free Fetal DNA Testing: Advantages, Challenges and Limitations. Presentation: Virtual Conference, 22nd Annual Education Day on Blood Transfusion Issues, 2021-09-24. |
|
|
Lhevinne Ciurcovich. Immunohematology Case Studies. Presentation: Immucor ImmuTECH Education Day (Virtual) 2021-05-05. |
|
|
Lynnette Beaudin, Dr. Lani Lieberman MD, FRCP, Fetal and neonatal alloimmune thrombocytopenia (FNAIT): Diagnosis, Investigation and Treatment. Presentation: U of T Monthly Transfusion Rounds (Virtual) 2021-02-25 |
|
|
Bodnar M, Hannaford K, Montemayor-Garcia C, Hannon J. Blindspots in Immucor BioArray RHD Molecular BeadChip Test: A Review of Cases at Canadian Blood Services Referred Out for RHD Gene Sequencing. Poster/Abstract, CSTM 2021 |
|
|
Floch A, Vege S, Berardi P, Hannon J, Ochoa-Garay G, Lomas-Francis C, et al. A change in RHD is associated with aberrant transcription and very weak D phenotype. Transfusion 2021 |
|
|
Flegel WA, Bodnar M, Clarke G, Hannon J, Lieberman L., ‘What constitutes the most cautious approach for a pregnant person with weak D type 4.0?’ Letter to the Editor, CMAJ June 2021 |
