Previous posts in our "The things we do for safety" series
In a previous post, we wrote about the donor screening criteria, the arm scrub and the diversion pouch. These are important steps in our blood collection process to limit the introduction of bacteria present on the skin or in the blood of donors into blood products. In this post, we explore other lines of defence in our battle against bacteria.
The entire blood product manufacturing process is conducted in a “closed-system”. Through a clever set-up of tubings and bags, the collected blood is never exposed to the external environment (containing millions of bacteria) during the production process. That is why the industry has developed the intricate collection bag that you will see when you come to our clinics to donate. We have come a long way from the glass collection bottles that were used in the early days of transfusion medicine. Our extensive quality system ensures that our products are regularly tested for bacterial contamination.

But even with all of these lines of defence, there is still risk that the blood product could be contaminated. So a visual check is performed on all blood products. While a single bacterium is invisible to the human eye, when they grow to large numbers they sometimes leave traces of their presence that are unmistakable to a trained specialist.
Staff in our organization and at hospitals are trained to do this visual inspection before blood components are shipped to hospitals and transfused. The most common signs would be an increased opacity of the product (bacteria in large numbers), the presence of excessive and unusual air bubbles (bacteria may produce gas), and presence of clots and fibrin strands (bacteria may activate the clotting process). We publish a Visual Assessment Guide to educate professionals on what to look for. Products showing those visual signs are not acceptable for transfusion and are discarded.
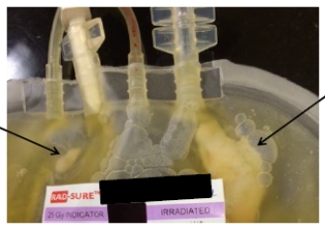
For platelet products we go one step-further. You may recall from our earlier blog about Platelets that this product must be stored at room temperature and in plasma, this makes for a particularly good environment for bacteria to grow. In 2004, Canadian Blood Services implemented the routine testing of all platelet units.

For every platelet product, we remove 8-10 mL which we add to a BacT/ALERT culture bottle — an environment that promotes bacteria growth if bacteria is present — and we monitor for bacteria growth over a few days. If bacteria is detected, then the platelet unit is discarded or recalled from the hospital. If additional products were made from the blood donation then those products are also recalled and discarded.
Dr. Sandra Ramirez, a development scientist at Canadian Blood Services’ Center for Innovation in Ottawa, is our in-house microbiology expert. She is working on a number of projects focused on bacteria and improving the safety and quality of blood products.
Further reading:
- ResearchUnit: How do bacteria behave during platelet production?
- ResearchUnit: Dodging disinfection: biofilm-forming skin bacteria can resist disinfectants.
- Practical Transfusion Medicine, Chapter 16 “Bacterial Contamination”
- Comparison of bacterial attachment to platelet bags with and without preconditioning with plasma. Loza-Correa M, Kalab M, Yi QL, Eltringham-Smith LJ, Sheffield WP, Ramirez-Arcos S. Vox Sang. 2017 May 3. doi: 10.1111/vox.12513.
- Bacteria can proliferate in thawed cryoprecipitate stored at room temperature for longer than 4 h. Ramirez-Arcos S, Jenkin C, Sheffield WP. Vox Sang. 2017 Apr 6. doi: 10.1111/vox.12517.
- Efficiency of riboflavin and ultraviolet light treatment against high levels of biofilm-derived Staphylococcus epidermidis in buffy coat platelet concentrates. Taha M, Culibrk B, Kalab M, Schubert P, Yi QL, Goodrich R, Ramirez-Arcos S. Vox Sang. 2017 Apr 5. doi: 10.1111/vox.12519.
Don't miss our two previous posts in "The things we do for safety" series which explored the first lines of defence in battling bacteria — donor screening criteria, the arm scrub and the diversion pouch — and leukoreduction — how white blood cells or leukocytes get filtered out of blood products to reduce the risk of transfusion-related reactions.
Canadian Blood Services – Driving world-class innovation
Through discovery, development and applied research, Canadian Blood Services drives world-class innovation in blood transfusion, cellular therapy and transplantation—bringing clarity and insight to an increasingly complex healthcare future. Our dedicated research team and extended network of partners engage in exploratory and applied research to create new knowledge, inform and enhance best practices, contribute to the development of new services and technologies, and build capacity through training and collaboration. Find out more about our research impact.
The opinions reflected in this post are those of the author and do not necessarily reflect the opinions of Canadian Blood Services nor do they reflect the views of Health Canada or any other funding agency.
Related blog posts
Canadian Blood Services implemented leukoreduction for the production of its red blood cells and platelet components in the late 1990s.
A previous post in our "The things we do for safety" series explores leukoreduction - or how white blood cells or leukocytes get filtered out of blood products to reduce the risk of transfusion-related reactions. Battling bacteria (part 1) Bacteria can be found pretty much everywhere and while some...