The images in this "through the microscope" are from recent work done to determine the best conditions to successfully perform the monocyte monolayer assay—a laboratory-based test that predicts the severity of adverse reactions to blood products. "Mastering the monocyte monolayer assay" is a new ResearchUnit that summarizes the study.
Through the microscope
From its first description in the 1980s, the monocyte monolayer assay has been modified and optimized. When performed correctly, the level of phagocytosis (ingestion) of red blood cells in the monocyte monolayer assay can help predict the clinical outcome of red blood cell destruction (hemolysis) in the patient.
Since this laboratory assay was first described, it has been shown to have excellent predictive capacity for which donor blood units transfused into patients with antibodies will survive normally. Recently, this assay has been used as the sole method to select appropriate, compatible blood for transfusion in difficult patients.
Hematological staining of cells in a monocyte monolayer assay performed using sub-optimal conditions
The image below shows ‘rosette’ formation, in which red blood cells (paler cells) bind to the periphery of the monocytes (darker cells), but the red blood cells are not phagocytosed (ingested) by the monocyte. This indicates that the monocytes recognize the red blood cells - via the Fc receptor of the antibody Fc region - but because the conditions are not optimal, there is NO phagocytosis.

Photo credit: Dr. Don Branch
Hematological staining of cells in a monocyte monolayer assay performed using optimal conditions
The image below shows red blood cells (paler cells) being engulfed and ingested (phagocytosed) by monocytes (darker cells) due to recognition by Fc receptor of the antibody Fc region. For phagocytosis to occur in a monocyte monolayer assay, optimal conditions of temperature (37°C) and pH (physiologic pH, maintained using buffers) are necessary to reproduce what would happen in a patient.
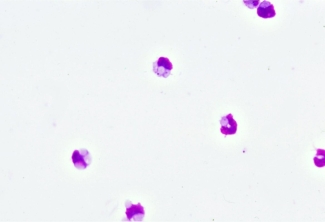
Photo credit: Dr. Don Branch
This assay was pioneered by Canadian Blood Services’ scientist, Dr. Don Branch, and has been used for over 35 years to predict the clinical outcome of incompatible blood transfusions. In recent years, it has been adapted to look at red blood cell destruction associated with IVIG treatment. Despite its long use, the optimal conditions for performing this assay have not been firmly established. In particular, the best way to prepare and store whole blood and monocytes for the monocyte monolayer assay has not been well studied.
What's a monocyte monolayer assay?
To predict whether a red blood cell transfusion is compatible, a laboratory test is used to see whether the patient’s antibody will react to donor’s red blood cell antigens. For some patients with antibodies, it can be very difficult to find compatible blood, and often the only choice is to transfuse the “least incompatible” blood. But how do you make that choice?
Unfortunately, looking at antibody-antigen reactions alone cannot predict whether the patient will have a clinically important reaction. This is because antibody binding is just the first step in a complex process in the body that leads to the red blood cell destruction (hemolysis) that can make the patient sick after an incompatible transfusion.
The “monocyte monolayer assay” is used to better predict the risk of a clinically significant reaction. Developed in the 1980s, this cell-based assay helps determine the risk of transfusing antigen-positive red blood cells to a patient who has an antibody or antibodies against the antigen(s).
How does it work?
Monocytes, the immune cells that destroy red blood cells during an adverse transfusion reaction, are isolated and placed in a single layer on a plate in the laboratory. The donor red blood cells are mixed with the patient’s antibodies and added to the monocytes.
Under a microscope, the number of monocytes with one or more red blood cells adhered/engulfed at the end of the assay is counted and this is used to calculate a “monocyte index”. If the monocyte index is less than 5 per cent, this indicates that the red blood cells won’t be rapidly destroyed and that the patient won’t have a serious reaction to the blood.
Further reading:
- Mastering the monocyte monolayer assay
- Graduate fellow, Cindy Tong, heads to Taiwan on a CIHR Travel Program Award
- Through the microscope: macrophage meets blood cell
- The wonder drug you’ve probably never heard of – yet
- Optimal conditions for the performance of a monocyte monolayer assay
Canadian Blood Services – Driving world-class innovation
Through discovery, development and applied research, Canadian Blood Services drives world-class innovation in blood transfusion, cellular therapy and transplantation—bringing clarity and insight to an increasingly complex healthcare future. Our dedicated research team and extended network of partners engage in exploratory and applied research to create new knowledge, inform and enhance best practices, contribute to the development of new services and technologies, and build capacity through training and collaboration.
The opinions reflected in this post are those of the author and do not necessarily reflect the opinions of Canadian Blood Services nor do they reflect the views of Health Canada or any other funding agency.
Related blog posts
Ms. Tong is a Canadian Blood Services' graduate fellowship program award recipient working with Dr. Don Branch in his Centre for Innovation lab in Toronto. She has also been selected to participate in a highly competitive Canadian Institutes for Health Sciences (CIHR) Travel Award Summer Program in...
Images from the Lazarus Research Group lab show some fascinating and potentially life-saving science in action.

