The difference (about) a day makes: extending the shelf life of platelets
In August 2017, Canadian Blood Services extended the shelf of our pooled and apheresis platelet components from five days to seven days. As part of this change, we implemented a new testing algorithm to improve our ability to detect bacterially contaminated pooled and apheresis platelet components to augment our current safety measures.
What did we do?
- We added an anaerobic culture bottle to our culture process to improve our ability to detect anaerobic bacteria. This doubled the sample volume size and increased our ability to detect aerobic microbes that can grow in anaerobic conditions.
- We increased the time of sample collection for bacterial detection from 24 to 36 hours post component collection. The increased time allows potentially contaminating bacteria a longer period of time to proliferate, thus improving our ability to detect them.
- We added a six-hour hold prior to the release of platelets, thus allowing any components that may be flagged by our bacterial detection instrument (BacT/ALERT 3D) early in the incubation period to be quarantined and not issued to a hospital customer.
In addition to the added safety for patients, we also expect to see a reduction in system-wide (nationally, at hospitals and at Canadian Blood Services) platelet outdates. Over the first few months after the implementation date, our hospital liaison specialist team discussed adjustments to hospital inventory levels and standing orders, and our production team adjusted their manufacturing targets. The early data indicates that we have been successful (see graph below). Note: the February data showing below was generated before all hospitals had shared their February disposition data.
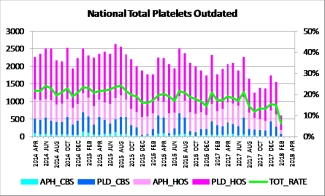
|
Left axis – total doses outdated |
Right axis – percent doses outdated |
|
Aph_CBS – apheresis platelet outdated at CBS |
Aph_HOS – apheresis platelet outdated at hospitals |
|
PLD_CBS – pooled platelet outdated at CBS |
PLD_HOS – pooled platelet outdated at hospitals |
Given that it is still early days since implementation of extended shelf life platelets, and in consideration of other initiatives anticipated to impact platelet discards, we expect to continue to see further outdate reductions across the system. As with all product and process improvements, this change aims to deliver optimal use of blood donor’s gift, provide better service to hospital customers and the patients we serve, while being as cost effective as possible.