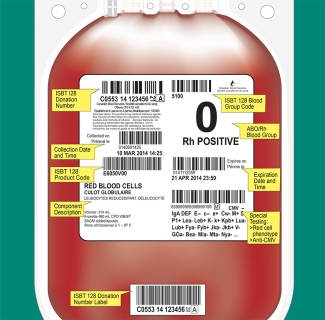
Label Format and Material
The Canadian Guidelines for the Uniform Labelling of Blood and Blood Components Using ISBT 128 describes the options selected by the Canadian blood operators, Canadian Blood Services and Héma-Québec for use in Canada and is intended to be used in conjunction with the ISBT 128 Standard: Technical Specification.
The following chart, along with the Canadian Guidelines for the Uniform Labelling of Blood and Blood Components Using ISBT 128, provides hospitals that are upgrading or implementing a laboratory information system with quick links to Customer Letters with relevant product, facility and ABO/Rh codes.
| PRODUCT CODE | FACILITY/SITE CODES | ABO/RH CODES |
|---|---|---|
|
List of most recent product codes. Report run date: |
List of /site most recent facility codes. | The list of ABO/Rh codes can be found in appendix 1 of the Canadian Guidelines for the Uniform Labelling of Blood and Blood Components Using ISBT 128. |